A new route to making graphene has been discovered that could make the 21st century’s wonder material easier to ramp up to industrial scale. Graphene — a tightly bound single layer of carbon atoms with super strength and the ability to conduct heat and electricity better than any other known material — has potential industrial uses that include flexible electronic displays, high-speed computing, stronger wind-turbine blades, and more-efficient solar cells, to name just a few under development.
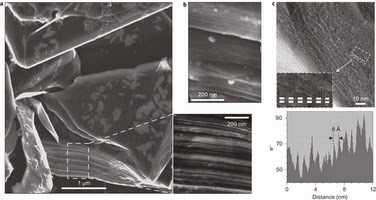
Edge-on electron microscopy images showing the expansion of graphite particles upon intercalation.
Nature Chemistry – Non-oxidative intercalation and exfoliation of graphite by Brønsted acids
n a paper first published online on Sept. 9 in the journal Nature Chemistry, Mallouk and colleagues at Penn State and the Research Center for Exotic Nanocarbons at Shinshu University, Japan, describe a method called intercalation, in which guest molecules or ions are inserted between the carbon layers of graphite to pull the single sheets apart.
The intercalation of graphite was achieved in 1841, but always with a strong oxidizing or reducing agent that damaged the desirable properties of the material. One of the most widely used methods to intercalate graphite by oxidation was developed in 1999 by Nina Kovtyukhova, a research associate in Mallouk’s lab.
While studying other layered materials, Mallouk asked Kovtyukhova to use her method, which requires a strong oxidizing agent and a mixture of acids, to open up single layers of solid boron nitride, a compound with a structure similar to graphite. To their surprise, she was able to get all of the layers to open up. In subsequent control experiments, Kovtyukhova tried leaving out various agents and found that the oxidizing agent wasn’t necessary for the reaction to take place.
Mallouk asked her to try a similar experiment without the oxidizing agent on graphite, but aware of the extensive literature saying that the oxidizing agent was required, Kovtyukhova balked.
“I kept asking her to try it and she kept saying no,” Mallouk said. “Finally, we made a bet, and to make it interesting I gave her odds. If the reaction didn’t work I would owe her $100, and if it did she would owe me $10. I have the ten dollar bill on my wall with a nice Post-it note from Nina complimenting my chemical intuition.”
Mallouk believes the results of this new understanding of intercalation in boron nitride and graphene could apply to many other layered materials of interest to researchers in the Penn State Center for Two-Dimensional and Layered Materials who are investigating what are referred to as “Materials Beyond Graphene.” The next step for Mallouk and colleagues will be to figure out how to speed the reaction up in order to scale up production
Abstract
Graphite intercalation compounds are formed by inserting guest molecules or ions between sp2-bonded carbon layers. These compounds are interesting as synthetic metals and as precursors to graphene. For many decades it has been thought that graphite intercalation must involve host–guest charge transfer, resulting in partial oxidation, reduction or covalent modification of the graphene sheets. Here, we revisit this concept and show that graphite can be reversibly intercalated by non-oxidizing Brønsted acids (phosphoric, sulfuric, dichloroacetic and alkylsulfonic acids). The products are mixtures of graphite and first-stage intercalation compounds. X-ray photoelectron and vibrational spectra indicate that the graphene layers are not oxidized or reduced in the intercalation process. These observations are supported by density functional theory calculations, which indicate a dipolar interaction between the guest molecules and the polarizable graphene sheets. The intercalated graphites readily exfoliate in dimethylformamide to give suspensions of crystalline single- and few-layer graphene sheets.
If you liked this article, please give it a quick review on ycombinator or StumbleUpon. Thanks

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.