For some infectious diseases, traditional vaccines just don’t cut it. Microbes that hide inside human cells and cause chronic illness aren’t stymied by the antibody response generated by the kind of vaccine available at the doctor’s office. T-cell vaccines, which activate a different type of immune response, could, in theory, better prevent or control such chronic infections, but so far nobody has been successful at transitioning T-cell vaccines from the lab bench to the clinic.
A Cambridge, Massachusetts, biotech company called Genocea thinks its high-throughput method could change that. The company will begin its first clinical trial later this year, when its experimental herpes vaccine will be the first test of its claims.
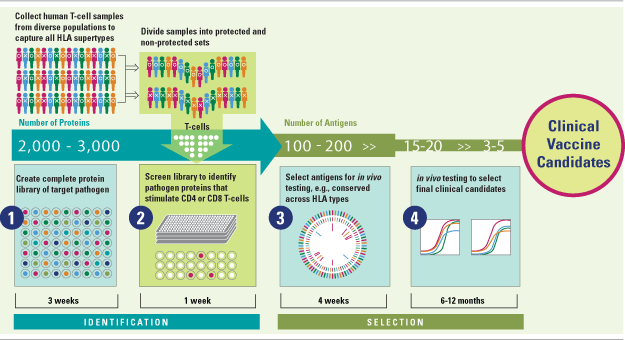
Genocea’s T Cell Antigen Discovery Technology: AnTigen Lead Acquisition System (ATLAS™)
Genocea’s vaccine programs are built around a transformational platform for the rapid discovery of T cell antigens. T cell antigens, specifically antigens that stimulate CD4+ and CD8+ T cells,are critical to generating disease‐specific cellular immune responses and long-term T cell memory.
Genocea’s approach addresses several key challenges to T cell antigen discovery, including identification of promising antigens from among the thousands of possible candidates for each pathogen and selection of antigens with potential to provide protection across diverse populations.
In less than three years, Genocea has taken four programs in three diseases from project initiation to animal proof-of-concept, a dramatically accelerated pace compared with up to 10-12 years with traditional discovery approaches. The technology is based on pioneering research by Darren Higgins, Ph.D., while at The University of California at Berkeley and Harvard Medical School.
High Throughput Approach Mimics Natural Immune Response
At the core of ATLAS™ is a high throughput screening process that mimics the natural mammalian immune response to protein antigens, including antigen processing and presentation by antigen presenting cells (APCs); CD4+ and CD8+ T cell recognition of APC-displayed peptides; and immune activation. Critically, the company screens all of a pathogen’s proteins against T cells obtained from human donors with diverse HLA types who have either generated a potentially protective or ineffective immune response after exposure to a target pathogen. As part of its discovery approach the company creates a library of each pathogen’s complete proteome.
As a result, ATLAS™ winnows what can be as many as several thousand proteins in a pathogen proteome down to a set of a small number of protein antigens that correlate with natural immunity. A subset of proteins from that group is selected for in vivo testing, based on recognition across multiple HLA supertypes and other criteria, with the goal of identifying two to five antigens for formulation and development into a vaccine candidate that will be protective across diverse ethnic populations.
All existing vaccines rouse the body into creating antibodies that attach to the surface of infecting microbes and flag them for destruction. But pathogens that live inside our cells, such as the viruses, bacteria, and other microbes that cause AIDS, malaria, herpes, and chlamydia, can evade this surveillance. “In order to deal with those types of pathogens, oftentimes we have to stimulate what we call cellular immunity. Unlike antibody immunity, which recognizes pathogens directly, cellular immunity has to recognize the infected cell and get rid of your own infected cells,” says Darren Higgins, a biologist at Harvard Medical School who studies the interaction between hosts and pathogens and is a cofounder of Genocea.
But activating cellular immunity—and the T cells that drive it—is challenging. The trial-and-error method used to develop antibody-based vaccines has not worked for T-cell vaccines. Despite years of academic and industry work, and even clinical trials, there are no T-cell vaccines for infectious disease on the market. “We don’t know all of the rules yet if it’s possible to make a T-cell vaccine, [nor] how effective it would be,” says Robert Brunham, a physician-scientist at the University of British Columbia in Vancouver who is also working on developing a chlamydia vaccine.
Indeed, our understanding of how T cells control infection is still developing. The challenge is to identify the right protein—or antigen—from a pathogen that will grab a T cell’s attention and signal that a human cell harbors an infectious agent. “If you can figure out what those protein pieces are, then you can use those proteins as a vaccine to sort of educate your immune system on what to respond to,” says Higgins, who is now a consultant and scientific advisor for Genocea.
The size of the challenge depends on the number of proteins encoded by a pathogen’s genome. Each of the 80 or so proteins in the herpes simplex 2 genome is a possibility, as are the thousand or so proteins in chlamydia and the 5,000 or so in malaria. Testing each protein one by one is a slow and expensive process. Genocea’s approach involves collecting as many of the pathogen’s proteins as can reasonably be produced in a lab, and then monitoring how human immune cells respond to each.
Generally, this involves isolating two kinds of immune cells from people—T cells and antigen-presenting cells, which carry bits of bacteria or other pathogens on their outer surface to display them to T cells. If a T cell produces immune-signaling molecules in response to a particular antigen, researchers at Genocea consider that antigen to be a potential vaccine candidate. By screening through nearly all of a pathogen’s proteins in its initial hunt for good vaccine candidates, the company thinks it can reduce the amount of time and money needed to develop a T-cell vaccine.
But there’s another layer of complication to the T-cell response that requires further refining to the vaccine candidate pool: human genetics. A protein that elicits a response in one person may not work in another, because there is genetic variety in the structures that antigen-presenting cells use to hold up antigens. “Whether that’s a barrier to having a universal vaccine or not is something the field is working through,” says Brunham. Genocea hopes to approach this problem by testing T-cell responses in immune cells from a variety of genetic backgrounds.
Genocea plans to enter clinical trials with its genital herpes vaccine later this year. If successful, Genocea’s herpes simplex 2 vaccine would be the first to combat the disease, which affects one out of every six people aged 15 to 49. Currently, patients can take antiviral drugs as a treatment, but there is no cure. Genocea’s candidate vaccine would be used as a therapeutic treatment for patients who already have the disease.
Genocea’s herpes vaccine program is moving faster than typical vaccine research, which can take 10 years to go from discovery to proof-of-concept and 20 years to reach the market, says Higgins. “Now you can screen very rapidly what is going to be the optimal vaccine component that allows you to get into clinical trials at a rapid rate.”
Pneumonia
The company has made rapid, significant progress discovering human antigens that elicit Th17 responses and protect against pneumococcal colonization.
Although infants in the U.S. are routinely immunized, the high cost makes the conjugate vaccines inaccessible in much of the developing world, where most deaths occur. In addition, the current vaccines only protect against a few of the nearly 100 types of pneumococcus and do not cover many of the known strains. Genocea’s program, which is funded through a partnership with the global non-profit organization, PATH, in collaboration with Children’s Hospital Boston, is aimed at developing an affordable, pneumococcal vaccine that provides broad coverage across bacterial strains.
Worldwide, S. pneumoniae infection is the leading cause of death for children under five; it is responsible for approximately 1.2 million deaths annually. Current estimates indicate that 150 million episodes of pneumonia occur every year among children less than five in developing countries.
Malaria
According to the World Health Organization (WHO), malaria, a mosquito-borne disease caused by the Plasmodium parasite, affected 170-311 million people in 2008 and caused more than 860,000 deaths. According to the WHO, half of the world’s population lives in areas at risk of malaria transmission. The disease produces fever, chills, and flu-like symptoms.
The disease is treatable with drugs, but there is no approved vaccine for malaria, widely regarded as the best solution for controlling the disease
Herpes Simplex Virus 2
Genocea is developing both therapeutic and prophylactic vaccines for HSV-2 based on the discovery of antigens with technology. In preclinical proof-of-concept studies, the antigens have demonstrated protection against disease, reduced viral shedding and lesion recurrence.
HSV-2 infection can cause recurring, painful genital sores, and can be stigmatizing and produce considerable psychological distress in patients. The disease is particularly severe in immunosuppressed patients and poses significant risk to newborns if it is transmitted from mothers during birth.
Herpes simplex virus type 2, (HSV-2), the most common cause of genital herpes, is a sexually transmitted disease that is estimated to infect more than 500 million people worldwide and one out of six people aged 15 to 49. In the U.S. alone, an estimated 50-60 million people are affected. There is neither a cure nor a vaccine to prevent or treat HSV-2. Antivirals are currently used to treat acute outbreaks and reduce transmission risk.
If you liked this article, please give it a quick review on ycombinator or StumbleUpon. Thanks

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.