SENS is a antiaging project. They report an increase in funding of about 40% from 2010 to 2011. They were able to double internal research levels. The funding would ideally be at levels that are 12 to 60 times higher ($20-100 million per year).
Here is a link for donating online to SENS
SENS Foundation annual report for 2011 (12 pages)
SENS foundation research report for 2011 (20 pages)
SENS Foundation had income of $1,507,000 in 2011. We greatly appreciate the support of the many individuals who contributed to our mission. We would like to thank Peter Thiel, Jason Hope, the Methuselah Foundation, and all of our contributors and volunteers for their on-going generosity. We expect a significant increase in both revenues and expenses for 2012, as we begin to see distributions from a de Grey family trust, under a grant from SENSF-UK. This support will be in addition to the contributions we receive from other sources.
SENS Foundation was able to make expenditures of $1,518,000 in 2011. This was an increase of over $400,000 from 2010, overwhelmingly in support of direct research and conference projects. We doubled our investment in our internal research capabilities, expanding the facility itself, adding capital equipment available for performing research, and increasing staff. With the 2011 addition of four new collaborations, we also laid the groundwork for a similar expansion of our extramural research programs in 2012.
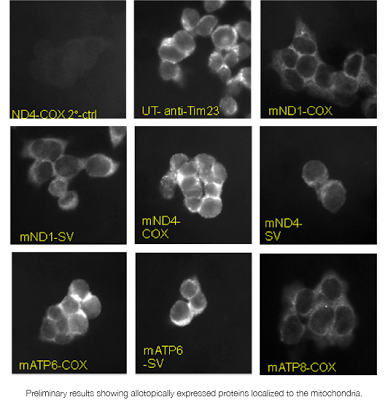
SENSF-RC: MitoSENS
Thirteen proteins critical to the respiratory chain are encoded only in mitochondrial DNA. However, nuclear DNA is much less susceptible to damage and is more easily repaired. The goal of this project is to test whether these thirteen genes, when encoded in the nucleus, can successfully be expressed and integrated into the mitochondria.
In 2011, in-house researchers Dr. Matthew “Oki” O’Connor and Dr. Gayathri Swaminathan successfully established multiple stable cell lines for each of the five modified mitochondrial genes with which they are working.
Heading into 2012, the team is beginning rigorous testing of the nuclear expression they observed in their five targeted mitochondrial genes.
Following that, they will analyze the complexes of the respiratory chain, which should demonstrate and confirm that the proteins encoded by these five genes have been integrated properly into the complexes.
If protein integration is confirmed, the team will move on to testing ways to functionally rejuvenate aged or otherwise damaged respiratory chain complexes. If successful, this approach could ultimately lead to novel ways of treating mitochondrial dysfunction — which has positive implications both for age-related disease and other mitochondrial disorders that can occur at any point in life.
SENSF-RC/Stanford : LysoSENS
Lysosomes are the cell’s last and best resort for degrading damaged or unwanted material. However, materials that the lysosome cannot degrade can form lipofuscin, filling the lysosome and causing it to lose its function.
The purpose of this project is to identify enzymes that can degrade A2E, a component of lipofuscin in retinal pigment epithelial (RPE) cells, and to successfully target those enzymes to the lysosomes of RPE cells.
The Research Center’s LysoSENS team, under the oversight of Dr. Gouri Yogalingam (jointly affiliated with SENS Foundation and Stanford University), has optimized protocols for the large-scale and rapid production and purification of A2E. Our new processes have led to verification of successful A2E degradation in vitro using two candidate enzymes, laccase and manganese peroxidase (MnP). We have also determined that recombinant MnP has the potential to be taken up by RPE cells effectively, thanks to its extensive mannosylation.
In the coming year, the team will test delivery of MnP to the lysosomes of A2E-loaded RPE cells by receptor-mediated endocytosis, a realistic therapeutic delivery mechanism. They will continue to express and purify other A2E-degrading enzymes, and to scale up enzyme pro-duction, as they have throughout the last year.
Extending Our Research Program
The in-depth project descriptions in the 20 page SENS foundation research report for 2011 tell only part of the story of SENS Foundation’s plans for 2012. We would thus like to conclude this report with a short preview of several exciting new efforts currently underway.
Thymus Project
We’ve begun a collaborative effort to create an artificial thymus, as part of our program to rejuvenate the immune system. This project will exploit the “decellularization” technology that others have used for heart, trachea and other organs in recent years, but for the purpose of rejuvenating the organ that suffers the most dramatic structural degeneration during human adulthood.
OncoSENS Expansion
We have initiated new intramural and extramural projects focusing on the implementation side of our OncoSENS program.
Specifically, our SENSF-RC team seeks to elucidate the genetic basis of ALT (Alternative Lengthening of Telomeres),
the mysterious telomerase-independent method that 10% of human cancers use to maintain telomere length. Meanwhile, a planned collaborative effort with one of our extramural partners will explore the ability of circulating stem cells to infiltrate the gut wall and repopulate the intestinal lining when stem cells there are failing, a problem that is expected to occur as a side-effect of the OncoSENS anti-cancer strategy.
RepleniSENS Development
Our RepleniSENS program is likewise moving forward. Stem cell therapy often works less well in older organisms, and this may largely be due to changes to the composition of the plasma. A multi-site project involving several prestigious laboratories is set to explore the impact of a rejuvenated circulation on improving the effectiveness of delivering stem cells to a variety of tissues in the elderly.
Nematode Longevity
While the majority of our efforts remain directed toward testing and development per the SENS proposal (as we believe this plan to have the best chance of delivering the comprehensive damage-repair therapies necessary to effectively mitigate the diseases of aging), we remain open to other possible strategies that might prove effective. We are therefore initiating a project to determine whether a recent spectacular longevity result in nematodes is actually as remarkable as it seems by revisiting some older work which may have underestimated the malleability of nematode longevity in response to famine.
Moving Toward an Improvised Mouse Model
We are getting closer to where the various genetic interventions we are currently exploring in cell culture can move to live mice. Projects involving genetic manipulation of mice are by nature lengthy efforts, and those attempting to intervene in aging are even more time-consuming, for obvious reasons. If a mouse could undergo significant genetic modification after reaching adulthood, this could hugely accelerate the pace of productive experiments and, accordingly, contract the timeline needed to gather critical data regarding the effectiveness of applied interventions. Within the next few months we will begin a project to investigate an intriguing newly-discovered approach to possibly achieving this.
2013 and Beyond
Given the tremendous recent increase in the breadth of SENS Foundation’s research efforts, we plan to focus the majority of our energies and resources over the next few years into driving existing projects beyond the “entry level” status that most of them occupy now. We will be redoubling our fundraising efforts so as to allow that process to occur as rapidly and productively as possible.
If you liked this article, please give it a quick review on ycombinator or StumbleUpon. Thanks

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.