Catalysts made of carbon nanotubes dipped in a polymer solution equal the energy output and otherwise outperform platinum catalysts in fuel cells, a team of Case Western Reserve University engineers has found. Platinum, which represents at least a quarter of the cost of fuel cells, currently sells for about $65,000 per kilogram. These researchers say their activated carbon nanotubes cost about $100 per kilogram.
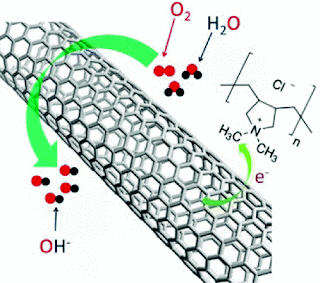
Having a strong electron-withdrawing ability, poly(diallyldimethylammonium chloride) (PDDA) was used to create net positive charge for carbon atoms in the nanotube carbon plane via intermolecular charge transfer. The resultant PDDA functionalized/adsorbed carbon nanotubes (CNTs), either in an aligned or nonaligned form, were demonstrated to act as metal-free catalysts for oxygen reduction reaction (ORR) in fuel cells with similar performance as Pt catalysts. The adsorption-induced intermolecular charge-transfer should provide a general approach to various carbon-based efficient metal-free ORR catalysts for oxygen reduction in fuel cells, and even new catalytic materials for applications beyond fuel cells.
Dai and research associates Shuangyin Wang and Dingshan Yu found that by simply soaking carbon nanotubes in a water solution of the polymer polydiallyldimethylammoniumn chloride for a couple of hours, the polymer coats the nanotube surface and pulls an electron partially from the carbon, creating a net positive charge.
They placed the nanotubes on the cathode of an alkaline fuel cell. There, the charged material acts as a catalyst for the oxygen-reduction reaction that produces electricity while electrochemically combining hydrogen and oxygen.
In testing, the fuel cell produced as much power as an identical cell using a platinum catalyst.
But the activated nanotubes last longer and are more stable, the researchers said. Unlike platinum, the carbon-based catalyst: doesn’t lose catalytic activity and, therefore, efficiency, over time; isn’t fouled by carbon monooxide poising; and is free from the crossover effect with methanol. Methanol, a liquid fuel that’s easier to store and transport than hydrogen, reduces activity of a platinum catalyst when the fuel crosses over from the anode to the cathode in a fuel cell.
The new process builds on the Dai lab’s earlier work using nitrogen-doped carbon nanotubes as a catalyst. In that process, nitrogen, which was chemically bonded to the carbon, pulled electron partially from the carbon to create a charge. Testing showed the doped tubes tripled the energy output of platinum.
Dai said the new process is far simpler and cheaper than using nitrogen-doped carbon nanotubes and he’s confident his lab will increase the energy output as well. “We have not optimized the system yet.”
7 pages of supplemental information
If you liked this article, please give it a quick review on ycombinator or StumbleUpon. Thanks

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.