Department of Energy’s ARPA-E selects 37 projects to pursue breakthroughs that could fundamentally change the way we use and produce energy. This is the first round of projects funded under ARPA-E, which is receiving total of $400 million under the American Recovery and Reinvestment Act.
Five of the 37 Projects are for Carbon Capture
Carbon capture projects
Chemical Looping Could Produce Clean Electicity and Hydrogen from Coal and Biomass
Pilot Scale Testing of Carbon Negative, Product Flexible Syngas Chemical Looping. A novel process known as Syngas Chemical Looping (SCL), in which coal and biomass are converted to electricity and CO2 is efficiently captured, has been successfully demonstrated on a laboratory scale. In this project, the SCL process, will be scaled up to a 250 kW pilot plant for a planned demonstration at the National Carbon Capture Center. Teaming with Ohio State University are PSRI, CONSOL Energy, Shell/CRI, and Babcock and Wilcox to accelerate this technology towards commercialization and deployment. (DOE grant: $5,000,000)
(36 page pdf) Syngas Chemical Looping: Particle Production Scale Up and Kinetics Investigation
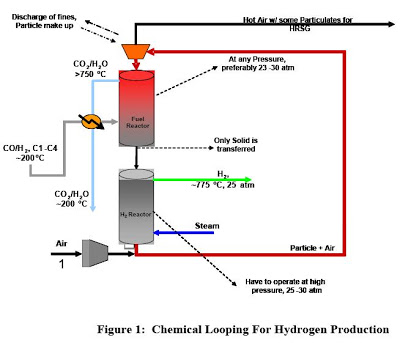
The syngas chemical looping process (SCL) is a novel method for the conversion of carbonaceous fuels to both electricity and hydrogen while capturing carbon dioxide and other pollutants. The SCL process has the potential to transform the conventional coal conversion processes to a clean, zero emissions process. The separation of CO2 and other contaminants is inherent in SCL, hence no dedicated pollutant control device is required. At the heart of the SCL process is an oxygen carrying metal oxide particle. The scale up of particle production was investigated because the total throughput of the process is directly proportional to the amount of particles being recycled. At present, particles are synthesized through pelletization of composite powders. The production rate of the particles was limited since the fine composite powders (2 – 7 microns) were constantly clogging during the feeding step. Through size increase of the composite powders to 425 – 1000 microns via granulation, clogging was significantly reduced. The scaled up process is currently limited by the size of the mixing drum. The maximum manageable amount of powder (lab scale test) is approximately 2 kg. A larger mixer of a style other than a rotary drum would be preferred.
An independent technical assessment (22 page pdf) of the potential of chemical looping in the context of a Fischer-Tropsch coal-to-liquids (CTL) plant. Several different concepts of chemical looping are being developed. In this analysis the concept under development by Ohio State University (OSU) was assessed to confirm that the thermochemical operations were in heat balance at temperatures compatible with an operable system, and to develop simulations of an entire coal to Fischer-Tropsch (F-T) liquids process, including the proposed looping scheme. Noblis was also asked to compare the technical performance results of a CTL plant with chemical looping with a conventional coal-to-liquids (CTL) system.
The Ohio State University (OSU) is developing a chemical looping scheme that could find application for treating tail gas from a coal based Fischer-Tropsch (F-T) Coal-to-Liquids (CTL) process. This chemical looping concept uses iron oxide (Fe2O3) to react with the unreacted synthesis gas (H2 and CO) and light hydrocarbons in the effluent tail gas from an F-T reactor. This reaction that takes place in a Fuel Reactor produces CO2, H2O and reduced iron. The reduced iron is then reacted with steam to produce hydrogen that can be recycled to the F-T reactor to adjust the input hydrogen to carbon monoxide ratio.
Membrane Process to Capture CO2 from Power Plant Flue Gas
CO2 Capture with Enzyme Synthetic Analogue. United Technologies Research Center (UTRC) will develop membrane technology for separating CO2 from flue gas streams using synthetic forms of carbonic anhydrase (CA), which natural systems use to manage CO2. Recent academic research has created synthetic analogue molecules for elucidation of CA enzyme mechanisms which are more robust in harsh environments. UTRC will team with Columbia University, CM-Tech, Hamilton Sundstrand and Worley Parsons in this program. (DOE Share: $2,251,183)
2 page fact sheet on Membrane Process to Capture CO2 from Power Plant Flue Gas
Pulverized coal (PC) plants burn coal in air to produce steam, and comprise 99% of all coal-fired power plants in the United States. CO2 is present in the flue gas exhaust at atmospheric pressure and a concentration of 10-15 volume percent.
The overall goal of this project is to demonstrate a cost-effective membrane-based process to capture CO2 from coal-fired power plant flue gas. The process will reduce power plant CO2 emissions and mitigate the potentially damaging effects of global warming. This project will provide a demonstration of CO2 capture from actual coal-fired flue gas with a membrane system using commercial-scale components. Results from this field test will provide key performance data to allow a thorough technical and economic evaluation of the proposed membrane process. The impact of system scale-up and the development of low-cost components on the capture process economics will be determined. The endpoint and primary technical objective of the program will be to complete a field test of MTR’s [Membrane Technology & Research] CO2 capture membrane process at a coal-fired power plant.
The objective of the proposed two-year research and development program is to develop, test, and validate a membrane process capable of effectively and efficiently capturing >90% of the CO2 from coal-fired power plant flue gas in the temperature range of 50-60 °C. The testing will include a slipstream field test of MTR’s membrane process using commercial modules to treat coal combustion flue gas.
More Energy Efficient CO2 Capture
Energy Efficient Capture of CO2 from Coal Flue Gas. Nalco and Argonne National Laboratory have partnered to develop an electrochemical process for CO2 capture. A technique known as Resin-Wafer Electrodeionization (RW-EDI) leverages control of pH to adsorb and desorb CO2 from flue gas without the need for heating or a vacuum. The objective is to drastically reduce the current parasitic power loss of 30% that is currently associated with carbon capture from flue gas. (DOE grant: $2,250,487)
2 page pdf on Resin-Wafer Electrodeionization
Carbon Nanotube Membranes
Carbon nanotube membranes for energy-efficient carbon sequestration. Porifera Inc will lead a team including the University of California and Lawrence Livermore National Laboratory that will integrate carbon nanotubes with polymer membranes to increase the flux of CO2 capture membranes by up to 100x. Physical and chemical modifications to the carbon nanotubes will be used to increase the selectivity of the membrane for CO2. The program objective is to demonstrate a more efficient and economical means of carbon capture over current state of the art amine technology. (DOE grant: $1,077,992)
Bakajin and Noy’s research originally focused on using carbon nanotubes as a less expensive solution to desalination. The technique involves a nanotube membrane on a silicon chip the size of a quarter that may offer a cheaper way to remove salt from water. The Livermore team created a membrane made of carbon nanotubes and silicon that may offer, among many possible applications, a less expensive desalination method.
Livermore’s carbon nanotubes will be integrated into polymer membranes to increase the flux of carbon dioxide capture membranes by two orders of magnitude. The technology could enable much less expensive carbon capture from coal plants.
Electric field swing adsorption for carbon capture applications. Electric Field Swing Adsorption (EFSA) is a technique that takes advantage of the ability of electric fields to change the interaction of molecules on a surface. In this project, Lehigh University will apply EFSA to high surface area conductive solid carbon sorbents for the adsorption and desorption of CO2 across a wide range of process conditions. The EFSA technique has the potential for drastically reduced parasitic load compared with current carbon capture methodologies. (DOE grant: $566,641)

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.