
Microphysiometer using multiwall carbon nanotubes is a major step toward developing the ability to assess the health of the body’s insulin-producing cells in real time.
Among other potential applications, this method could be used to improve the efficacy of a new procedure for treating Type 1 (juvenile) diabetes that has demonstrated the ability to free diabetics from insulin injections for several years. It works by transplanting insulin-producing cells into the pancreas of diabetics to replace the cells that the disease has disabled or destroyed.
One of the next steps is to use the microphysiometer to measure insulin, lactate and oxygen levels simultaneously. This will allow researchers to study how the islet cells react to the drugs and help identify the best way to deal with transplant rejection. It will also allow them to verify the health of the islets cells before they are transplanted into patients.
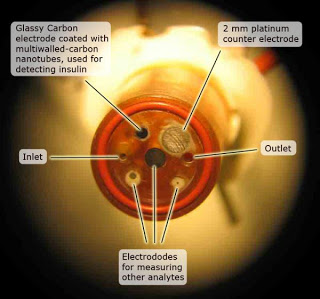
the researchers developed a new electrode for a device called a microphysiometer. The microphysiometer assesses the condition of living cells by submerging them in a saline solution, confining them in a very small chamber and then measuring variations in their metabolism. The volume of the chamber is only three microliters — about 1/20th the size of an ordinary raindrop — allowing the electrode to detect the minute amounts of insulin produced by special pancreatic cells called Islets of Langerhans.
The new electrode is built from multiwalled carbon nanotubes, which are like several flat sheets of carbon atoms stacked and rolled into very small tubes. Provided by William Hofmeister at the University of Tennessee Space Institute, the nanotubes are electrically conductive and the concentration of insulin in the chamber can be directly related to the current at the electrode and the nanotubes operate reliably at pH levels characteristic of living cells.
Current detection methods measure insulin production at intervals by periodically collecting small samples and measuring their insulin levels. The new sensor detects insulin levels continuously by measuring the transfer of electrons produced when insulin molecules oxidize in the presence of glucose. When the cells produce more insulin molecules, the current in the sensor increases and vice versa, allowing the researchers to monitor insulin concentrations in real time. It is similar to a device developed by another group of researchers that operated at acidity levels well beyond those where living cells can function.
Previous tests had shown that nanotube detectors are more sensitive at measuring insulin than conventional methods.
“One of the key advances of this project was finding how to keep nanotubes active on the surface without being washed away by microfluidic flows,” Cliffel says.
Now that the microphysiometer has demonstrated the ability to rapidly detect the small quantities of insulin produced by individual cells, the researchers hope to use it to determine the health of the islet cells used for transplantation.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.

Comments are closed.