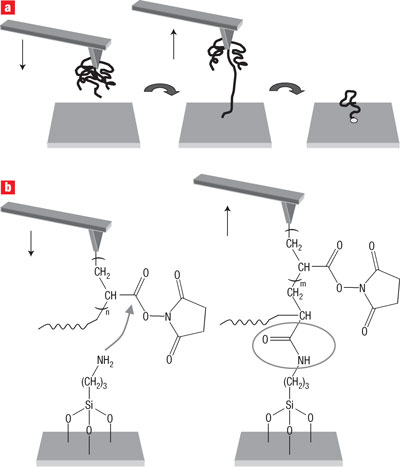
The use of scanning probe microscopy-based techniques to manipulate single molecules and deliver them in a precisely controlled manner to a specific target has been performed by a european research team. An atomic force
microscope (AFM) was used to deliver and immobilize single molecules, one at a time, on a surface. Reactive polymer molecules, attached at one end to an AFM tip, are brought into contact with a modified silicon substrate to which they become linked by a chemical reaction. When the AFM tip is pulled away from the surface, the resulting mechanical force causes the weakest bond — the one between the tip and polymer — to break. This process transfers the polymer molecule to the substrate where it can be modified by further chemical reactions.

a, Reactive polymer molecules, attached at one end to an AFM tip, are brought into contact with a substrate to which they can become linked by a chemical reaction. When the tip is pulled away from the surface, the resulting mechanical force causes the
weakest bond — the one between the tip and polymer — to break. b, During the tip–sample contact, a chemical reaction occurs between the activated esters of a
poly-N-succinimidyl acrylate chain grafted to the tip and the amino groups of the substrate to form an amide bond, which covalently links the chain to the substrate.
Gold-coated AFM tips were modified by electrografting poly-N-succinimidyl acrylate (PNSA), according to ref. 14. This electro-initiated polymerization is a convenient way to fabricate polymer brushes with a moderate grafting density and results in the direct chemisorption of the polymer onto the tip surface. Surfaces with amino functions were prepared by grafting aminopropyltrimethoxysilane to silicon substrates. The activated esters of the polymer can easily react, at room temperature, with the amino-derivatives. In an N,N-dimethylformamide (a good
solvent for PNSA) solution containing 4-dimethylaminopyridine (DMAP, a catalyst), the
functionalized AFM tip was slowly brought into contact with the surface. The chemical reaction between the PNSA activated esters and the amino groups of the
substrate forms amide bonds and covalently links polymer chains to the substrate (Fig. 1b). Upon retraction of the tip, single chains are stretched until a bond breaks. The Au(tip)–C(polymer) bond is the weakest link in the system and the most likely candidate for breaking. Upon cleavage, the polymer chain remains covalently attached to the substrate. The deposited chains are reactive and can be easily modified, subsequently, by a wide range of nucleophilic compounds.
The experiments strongly rely on the design and accurate modification of the tips. The use of a swollen brush with a low grafting density (less than one chain per 100 nm2; the compression profile shows that we are in the isolated mushrooms regime, see
Methods section) for force spectroscopy experiments prevents unspecific physisorption of a bulk three-dimensional structure onto the substrate thanks to steric repulsion and the strong adsorption of flat-lying chains (that adsorb through several attachment points) which leads to the appearance of multiple superimposed peaks in the force–distance curves. The presence of a good solvent prevents interactions within the polymer. Statistically, the bridging interactions should occur with only one isolated single polymer chain. The strategy makes the detection of the rupture of a single tip–polymer bond easy.

Brian Wang is a Futurist Thought Leader and a popular Science blogger with 1 million readers per month. His blog Nextbigfuture.com is ranked #1 Science News Blog. It covers many disruptive technology and trends including Space, Robotics, Artificial Intelligence, Medicine, Anti-aging Biotechnology, and Nanotechnology.
Known for identifying cutting edge technologies, he is currently a Co-Founder of a startup and fundraiser for high potential early-stage companies. He is the Head of Research for Allocations for deep technology investments and an Angel Investor at Space Angels.
A frequent speaker at corporations, he has been a TEDx speaker, a Singularity University speaker and guest at numerous interviews for radio and podcasts. He is open to public speaking and advising engagements.